11. Stochastic Calcium Burst model with GHK currents
This chapter is based on the publication “Anwar H, Hepburn I, Nedelescu H, Chen W, De Schutter E (2013) Stochastic Calcium Mechanisms Cause Dendritic Calcium Spike Variability. The Journal of Neuroscience, 33(40): 15848-15867”.
The corresponding python script: STEPS_Tutorial_CaBurst.py
This chapter builds on previous chapters by simulating a model that includes both a reaction-diffusion component as well as electrical excitability. As described in 1, the two are closely coupled and this model contains ion channels where activation is both voltage-dependent and calcium-dependent. In addition, the calcium ions form an important part of the current across the membrane, further coupling the reaction-diffusion component with the electrical excitability. This chapter introduces an important new object: the GHK Current object, which is described in some detail in section P-type Calcium channel.
In addition, we will also introduce the STEPS parameter system that can automatically generate parameter tables from python scripts.
As in previous chapters we will go through the script, looking in some depth at new concepts, but only brief explanations will be offered of things that have been described in previous chapters.
11.1. Modelling solution
At the start of the script, as usual, we will import some modules, including STEPS modules and some other python modules. We will first declare all important parameters for the module such as physical constants, membrane properties, kinetic properties of the channels, initial conditions and so on.
11.1.1. Parameters
As stated earlier, STEPS can automatically generate parameter tables from python scripts. In general, values that are used for setting properties of STEPS objects are registered as model parameters. At the end of a STEPS scripts, one can call e.g. ExportParameters(sim, '/path/to/fileprefix', method='csv') and STEPS will automatically generate csv files that contain the values and units of all parameters.
We start the script by import relevant modules and declaring a few parameters:
[1]:
import steps.interface
from steps.geom import *
from steps.model import *
from steps.rng import *
from steps.saving import *
from steps.sim import *
from steps.utils import *
import math
# # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # #
###########################################################
# Simulation Parameters
###########################################################
SEED = 1234
NBRUNS = 5
EF_DT = 5.0e-6
DT = 2.0e-5
ENDT = 0.5
###########################################################
# Model Parameters
###########################################################
TEMPERATURE = 34.0 + 273.15
Q10 = 3
FARADAY = 96485.3365 # C/mol
R = 8.3144621 # J/mol K
AVOGADRO = 6.02214129e23 # /mol
Qt = math.pow(Q10, (TEMPERATURE - (23 + 273.15)) / 10)
Qt_mslo = math.pow(Q10, (TEMPERATURE - (25 + 273.15))/10)
Note that since these parameters are standard python floating point numbers, we need to make sure all of them are expressed in SI units.
There is however another way to declare parameters that will allow us to specify units, keep track of the name of the parameter, and give additional information about the parameters. This is done by using the Parameter class from the utils module (see documentation):
[2]:
#######################################
# Membrane Parameters
#######################################
init_pot = Parameter(-60, 'mV', Description='Initial membrane potential')
Ra = Parameter(235.7*1.0e-2, 'ohm m', Description='Bulk resistivity')
memb_capac = Parameter(1.5e-2, 'F m^-2', Description='Membrane capacitance')
The Parameter constructor usually takes at least two arguments: the first one is the value of the parameter and the second one is the unit this value is expressed in. As can be seen in the above code, we can provide non-SI units and values; STEPS will then do the proper conversions automatically. When using the Parameter class, STEPS will use the name of the veriable (init_pot, Ra, etc.) as the name of the parameter in parameter tables. A list of supported units is available in
the Parameter class documentation.
The Parameter constructor can then take any keyword argument as additional information about the parameter. Here we supply a description of the parameter that will be displayed in the automatically generated parameter tables.
If a Parameter object is involved in computations, all other values involved in the computation should be Parameters as well, otherwise STEPS cannot infer the units of the value that results from the computation. Because of this constraint, we do not use the Parameter class everywhere:
[3]:
#######################################
# CaP channels parameters
#######################################
CaP_P = Parameter(2.5e-2, 'um^3 s^-1', Description='CaP single channel permeability')
CaP_ro = Parameter(38, 'um^-2', Description='CaP channels density')
# Reaction rates
vhalfm = -29.458 # mV
cvm = 8.429 # mV
def minf_cap(mV):
vhalfm = -29.458
cvm = 8.429
return 1 / (1 + math.exp(-(mV - vhalfm) / cvm))
def tau_cap(mV):
if mV >= -40:
return 0.2702 + 1.1622 * math.exp(-(mV + 26.798) ** 2 / 164.19)
else:
return 0.6923 * math.exp(mV / 1089.372)
alpha_cap = VDepRate.Create(
lambda V: (minf_cap(V * 1e3) / tau_cap(V * 1e3)) * Qt * 1e3
)
beta_cap = VDepRate.Create(
lambda V: (1 - minf_cap(V * 1e3)) / tau_cap(V * 1e3) * Qt * 1e3
)
# Initial conditions
CaP_p = [0.92402, 0.073988, 0.0019748, 1.7569e-05]
Note that we use VDepRate.Create(...) instead of VDepRate(...) so that the resulting STEPS object is named after the variable it is attributed to.
The other parameters are declared in a similar way:
[4]:
#######################################
# CaT channels parameters
#######################################
CaT_P = Parameter(1.65e-2, 'um^3 s^-1', Description='CaT single channel permeability')
CaT_ro = Parameter(3.7576, 'um^-2', Description='CaT channels density')
# Reaction rates
def minf_cat(mV):
vhalfm = -52
cvm = -5
return 1 / (1 + math.exp((mV - vhalfm) / cvm))
def taum_cat(mV):
if mV > -90:
return 1 + 1 / (math.exp((mV + 40) / 9) + math.exp(-(mV + 102) / 18))
else:
return 1
def hinf_cat(mV):
vhalfh = -72
cvh = 7
return 1 / (1 + math.exp((mV - vhalfh) / cvh))
def tauh_cat(mV):
return (15 + 1 / (math.exp((mV + 32) / 7)))
alpham_cat = VDepRate.Create(lambda V: minf_cat(V * 1e3) / taum_cat(V * 1e3) * 1e3)
betam_cat = VDepRate.Create(lambda V: (1 - minf_cat(V * 1e3)) / taum_cat(V * 1e3) * 1e3)
alphah_cat = VDepRate.Create(lambda V: hinf_cat(V * 1e3) / tauh_cat(V * 1e3) * 1e3)
betah_cat = VDepRate.Create(lambda V: (1 - hinf_cat(V * 1e3)) / tauh_cat(V * 1e3) * 1e3)
# Initial conditions
CaT_p = [
[0.58661, 0.23687, 0.023912], # h0
[0.10564, 0.042658, 0.0043063], # h1
]
#######################################
# BK channels parameters
#######################################
BK_G = Parameter(210, 'pS', Description='BK single channel conductance')
BK_ro = Parameter(2.0238, 'um^-2', Description='BK channels density')
BK_rev = Parameter(-77, 'mV', Description='BK channel reversal potential')
# Reaction rates
#Units (1)
Qo = 0.73
Qc = -0.67
#Units (/s)
pf0 = 2.39
pf1 = 5.4918
pf2 = 24.6205
pf3 = 142.4546
pf4 = 211.0220
pb0 = 3936
pb1 = 687.3251
pb2 = 234.5875
pb3 = 103.2204
pb4 = 11.6581
#Units(/M)
k1 = 1.0e6
#Units(/s)
onoffrate = 1.0e3
L0 = 1806
#Units (M)
Kc = 8.63e-6
Ko = 0.6563e-6
BK_f = k1*onoffrate*Qt_mslo
BKo_b = Ko*k1*onoffrate*Qt_mslo
BKc_b = Kc*k1*onoffrate*Qt_mslo
BK_f0 = VDepRate.Create(
lambda V: pf0 * Qt_mslo * (math.exp((Qo * FARADAY * V) / (R * TEMPERATURE)))
)
BK_f1 = VDepRate.Create(
lambda V: pf1 * Qt_mslo * (math.exp((Qo * FARADAY * V) / (R * TEMPERATURE)))
)
BK_f2 = VDepRate.Create(
lambda V: pf2 * Qt_mslo * (math.exp((Qo * FARADAY * V) / (R * TEMPERATURE)))
)
BK_f3 = VDepRate.Create(
lambda V: pf3 * Qt_mslo * (math.exp((Qo * FARADAY * V) / (R * TEMPERATURE)))
)
BK_f4 = VDepRate.Create(
lambda V: pf4 * Qt_mslo * (math.exp((Qo * FARADAY * V) / (R * TEMPERATURE)))
)
BK_oc_f = [BK_f0, BK_f1, BK_f2, BK_f3, BK_f4]
BK_b0 = VDepRate.Create(
lambda V: pb0 * Qt_mslo * (math.exp((Qc * FARADAY * V) / (R * TEMPERATURE)))
)
BK_b1 = VDepRate.Create(
lambda V: pb1 * Qt_mslo * (math.exp((Qc * FARADAY * V) / (R * TEMPERATURE)))
)
BK_b2 = VDepRate.Create(
lambda V: pb2 * Qt_mslo * (math.exp((Qc * FARADAY * V) / (R * TEMPERATURE)))
)
BK_b3 = VDepRate.Create(
lambda V: pb3 * Qt_mslo * (math.exp((Qc * FARADAY * V) / (R * TEMPERATURE)))
)
BK_b4 = VDepRate.Create(
lambda V: pb4 * Qt_mslo * (math.exp((Qc * FARADAY * V) / (R * TEMPERATURE)))
)
BK_oc_b = [BK_b0, BK_b1, BK_b2, BK_b3, BK_b4]
# Initial conditions
BK_p = [
[0.99997, 4.3619e-07, 4.1713e-09, 4.4449e-11, 6.3132e-14],
[2.5202e-05, 1.1765e-06, 6.6148e-08, 2.4392e-09, 4.0981e-11],
]
#######################################
# SK channels parameters
#######################################
SK_G = Parameter(10, 'pS', Description='SK single channel conductance')
SK_ro = Parameter(0.31, 'um^-2', Description='SK channels density')
SK_rev = Parameter(-77, 'mV', Description='SK channel reversal potential')
# Reaction rates
#Units (/s)
invc1 = 80
invc2 = 80
invc3 = 200
invo1 = 1000
invo2 = 100
diro1 = 160
diro2 = 1200
#Units ( /s M)
dirc2 = 200e6
dirc3 = 160e6
dirc4 = 80e6
invc1_t = invc1*Qt
invc2_t = invc2*Qt
invc3_t = invc3*Qt
invo1_t = invo1*Qt
invo2_t = invo2*Qt
diro1_t = diro1*Qt
diro2_t = diro2*Qt
dirc2_t = dirc2*Qt/3.0
dirc3_t = dirc3*Qt/3.0
dirc4_t = dirc4*Qt/3.0
# Intital conditions
SK_C1_p= 0.96256
SK_C2_p= 0.036096
SK_C3_p= 0.0010829
SK_C4_p= 6.4973e-06
SK_O1_p= 0.00017326
SK_O2_p= 7.7967e-05
#######################################
# Leak channels parameters
#######################################
L_G = Parameter(0.04, 'pS', Description='Leak single channel conductance')
L_ro = Parameter(0.25, 'um^-2', Description='Leak channels density')
L_rev = Parameter(-61, 'mV', Description='Leak channel reversal potential')
#######################################
# Ca pump channels parameters
#######################################
P_ro = Parameter(6.022141, 'um^-2', Description='Ca2+ pump density')
# Reaction rates
P_f = 3e9
P_b = 1.75e4
P_k = 7.255e4
#######################################
# Calcium buffering parameters
#######################################
# Ca concentrations
Ca_oconc = 2e-3
Ca_iconc = 45e-9
# Mg concentrations
Mg_conc = 590e-6
# Buffer concentrations
iCBsf_conc = 27.704e-6
iCBCaf_conc = 2.6372e-6
iCBsCa_conc= 1.5148e-6
iCBCaCa_conc= 0.14420e-6
CBsf_conc= 110.82e-6
CBCaf_conc= 10.549e-6
CBsCa_conc= 6.0595e-6
CBCaCa_conc= 0.57682e-6
PV_conc= 3.2066e-6
PVCa_conc= 16.252e-6
PVMg_conc= 60.541e-6
# Diffusion constants
DCST = 0.223e-9 # Ca
DCB = 0.028e-9 # Calbindin (CB)
DPV = 0.043e-9 # Parvalbumin (PV)
# Reaction rates
CBf_f_kcst = 4.35e7
CBf_b_kcst = 35.8
CBs_f_kcst = 0.55e7
CBs_b_kcst = 2.6
PVca_f = 10.7e7
PVca_b = 0.95
PVmg_f_kcst = 0.8e6
PVmg_b_kcst = 25
###########################################################
# Mesh Parameters
###########################################################
mesh_file = 'meshes/caburst_cyl80.msh'
11.2. Model specification
Since this is a relatively large model we will split its description up into several sections. We first create the species and channels that will be used in the model:
[5]:
###########################################################
# Biochemical model
###########################################################
mdl = Model()
with mdl:
# Species
Pump, CaPump, PV, PVMg, PVCa, Mg = Species.Create()
Ca = Species.Create(valence=2)
# Calbindin
CBs, CBf, CBsCa, CBfCa, CBmob, CBimmob = SubUnitState.Create()
CBsSU, CBfSU, CBmobSU = SubUnit.Create(
[CBs, CBsCa], [CBf, CBfCa], [CBmob, CBimmob]
)
CB = Complex.Create([CBsSU, CBfSU, CBmobSU], statesAsSpecies=True)
# Channels
CaPc, CaPo = SubUnitState.Create()
CaP_SU = SubUnit.Create([CaPc, CaPo])
CaPchan = Channel.Create([CaP_SU, CaP_SU, CaP_SU])
CaTmc, CaTmo, CaThc, CaTho = SubUnitState.Create()
CaTm_SU = SubUnit.Create([CaTmc, CaTmo])
CaTh_SU = SubUnit.Create([CaThc, CaTho])
CaTchan = Channel.Create([CaTm_SU, CaTm_SU, CaTh_SU])
BK, BKCa, BKopen, BKclose = SubUnitState.Create()
BKCaSU = SubUnit.Create([BK, BKCa])
BKocSU = SubUnit.Create([BKopen, BKclose])
BKchan = Channel.Create([BKCaSU, BKCaSU, BKCaSU, BKCaSU, BKocSU])
SK_C1, SK_C2, SK_C3, SK_C4, SK_O1, SK_O2 = SubUnitState.Create()
SKchan = Channel.Create([SK_C1, SK_C2, SK_C3, SK_C4, SK_O1, SK_O2])
Leak = SubUnitState.Create()
L = Channel.Create([Leak])
All ion channels involve the creation of SubUnitStates and SubUnits, as we already saw in the corresponding chapter. There is however one difference compared to previous chapters: the Ca species is created with the valence keyword parameter. This sets the net elementary electrical charge per calcium ion, which in this example for Ca2+ is +2. This is necessary for using GHK currents as we will see later in this chapter.
11.2.1. Calcium dynamics
The following lines of code describe the calcium and calcium buffer reactions and diffusion. Since these are ‘ordinary’ dynamics with no voltage-dependence we will not look look at this part in detail. A more detailed explanation is offered in 1 and 2.
[6]:
with mdl:
r = ReactionManager()
vsys = VolumeSystem.Create()
with vsys:
# PVCa
PV + Ca <r[1]> PVCa
r[1].K = PVca_f, PVca_b
# PVMg
PV + Mg <r[1]> PVMg
r[1].K = PVmg_f_kcst, PVmg_b_kcst
with CB[...]:
# Fast binding
CBf + Ca <r[1]> CBfCa
r[1].K = CBf_f_kcst, CBf_b_kcst
# Slow binding
CBs + Ca <r[1]> CBsCa
r[1].K = CBs_f_kcst, CBs_b_kcst
diff_Ca = Diffusion.Create(Ca, DCST)
diff_CB = Diffusion.Create(CB[:, :, CBmob], DCB)
diff_PV = Diffusion.Create(PV, DPV)
diff_PVCa = Diffusion.Create(PVCa, DPV)
diff_PVMg = Diffusion.Create(PVMg, DPV)
ssys = SurfaceSystem.Create()
with ssys:
# Ca Pump
Pump.s + Ca.i <r[1]> CaPump.s >r[2]> Pump.s
r[1].K = P_f, P_b
r[2].K = P_k
11.2.2. P-type Calcium channel
The P-type calcium channel is a different type of ion channel to those we have seen before. In previous chapters we saw Hodgkin-Huxley sodium and potassium channels that conducted an Ohmic current. The sodium and potassium ions in that situation were not explicitly simulated, which was reasonable because those ions were not involved in other processes we were interested in, and we could assume their concentrations inside and outside the cell were not altered significantly during their conduction. However, with calcium we need a different approach. Here calcium is involved in intracellular processes such as potassium channel-activation (as we will see), buffering and diffusion, and so we must simulate the influx of calcium through these P-type channels. Furthermore, the Ohmic approximation is no longer sufficient for our purposes. The large differences between intracellular and extracellular concentration along with large changes in intracellular concentration mean that, in effect, channel conductance has some voltage and concentration dependence and is described much better by the GHK flux equation. The GHK flux equation itself is derived under certain simplifying assumptions that are good approximations for many ion channels, specifically those where channel occupancy and competition are negligible. Please see 3 for further discussion on the use of the GHK flux equation and the behaviour of the GHK current object in STEPS. It is worth noting that use of the GHK flux equation means that (instead of conductance) we must specify the channel’s permeability, which can be more difficult to parameterize.
The P-type calcium channel kinetics are described in detail in 1. The CaPchan channel object was created before and contains three identical CaP_SU subunits that can either be in closed (CaPc) or open (CaPo) states.
We then declare the voltage-dependent transition reaction between open and closed states. Remember for each of these discrete channels the voltage will be read from the local voltage across the membrane triangle where the channel resides.
[7]:
with mdl:
with ssys:
# CaP channel
with CaPchan[...]:
CaPc.s <r[1]> CaPo.s
r[1].K = alpha_cap, beta_cap
We come to creating our GHK current object. This object will calculate single-channel current for a given channel state by the GHK flux equation:
where \(I_{s}\) is the single-channel current (amps) of ion S, \(P_{s}\) is the single-channel permeability of ion S (\(m^{3}.s^{-1}\)), \(z_{s}\) is the valence of ion S, \(V_{m}\) is the membrane voltage (volts), \(F\) is the Faraday constant, \(R\) is the gas constant, \(T\) is temperature (Kelvin), \([S]_{i}\) is the intracellular concentration of ion S (\(mol.m^{-3}\)) and \([S]_{o}\) is the extracellular concentration of ion S (\(mol.m^{-3}\)).
When a GHK current is applied in STEPS it (optionally) results in movement of ions between the ‘outer’ and ‘inner’ compartments, the direction of which will depend on the sign of the current and the valence of the ions.
Many of the values required for calculating a GHK current are simulation variables, such as concentrations and voltage, simulation constants such as temperature, or fixed constants such as the Faraday constant and the gas constant. Such values are either known or can be found by STEPS during runtime and so are not part of object construction, with the exception of single-channel permeability which we will come to later. Like we saw in Simulating membrane
potential, we then create the GHKCurr object. There are also optional keyword arguments (virtual_oconc and computeflux) that we will explain further.
[8]:
with mdl:
with ssys:
OC_CaP = GHKCurr.Create(
CaPchan[CaPo, CaPo, CaPo], Ca, CaP_P,
computeflux=True,
virtual_oconc=Ca_oconc,
)
First let’s look at the virtual_oconc argument. This option allows us to not explicitly model the extracellular (‘outer’) concentration of the ion, which is useful because often the extracellular compartment is not modelled. This option, rather, allows a fixed ‘outer’ concentration for the ion to be specified and that number will be used in the GHK flux calculations. The value of the parameter Ca_oconc that we declared earlier is 2mM.
The other optional argument is computeflux. This flag (which defaults to True) tells STEPS whether to model this GHK current process as ion transport or not. If computeflux is True, then the calculated GHK current will result in transport of ions between the ‘outer’ and ‘inner’ compartments. For example, if over some 0.01ms time step, somewhere on the membrane a mean current of approximately 1.6pA is calculated through a membrane channel to which a GHK current is applied, then for an ion
of valence 2+ this means that 50 ions moved from one compartment to the other. The direction of movement depends on the signs of the current and the ion valence. The movement only occurs between surface tetrahedrons surrounding the membrane triangles in which the channels reside and so, for ions where this kind of process occurs, for accuracy it is necessary to model diffusion of these ions at least within the inner compartment and often within both compartments. This can be an expensive
computation, particularly where concentrations are in the millimolar range, which shows the value of the computeflux flag- if the GHK flux is applied to an ion which does not have any other particularly important effects in the model other than its effect on membrane excitability (a possible example is potassium) then it may be a good labour-saver to clamp ‘inner’ and ‘outer’ concentrations of the ion and turn off the transport of ions as an approximation. However, in this model if we set
computeflux to False then the result would be no intracellular calcium, which is obviously not desirable, and so the computeflux flag is set to True, as it usually will be for most ions in most models.
The first positional argument, like for ohmic currents, is the conducting channel state(s). Here, the channel opens when all its subunits are in the open CaPo state.
The second positional argument is the ion that will carry the current. For calcium (and only for calcium) we used valence=2 to specify a valence of 2. ‘Valence’ can be an ambiguous term, but here it means the net elementary electrical charge per ion, which in this example for Ca2+ is +2. Negative valences can of course be specified by using a negative number. It is essential that a valence is set for any ion that will be used for a GHK current in the simulation. If no valence is specified
the result will be an error.
The third positional argument (CaP_P) corresponds to single-channel permeability. Because conductance is not constant for a GHK current (apart from under certain unusual conditions) one value for a conductance parameter does not suffice. However, since single-channel permeability is often rather a difficult parameter to define, STEPS does provide functionality for estimating the permeability. So we have two options for setting single-channel permeability: giving it directly to the GHKCurr
constructor; or giving the result of a call to steps.API_2.model.GHKCurr.PInfo to estimate the permeability from data. The first is straightforward and simply means providing single-channel permeability in S.I. units of cubic metres / second. In this model the CaP channel permeability CaP_P was set to 2.5e-20 cubic metres / second 4. If we did not provide the permeability during construction, we could also write
OC_CaP.P = CaP_P
The second option, the steps.API_2.model.GHKCurr.PInfo function, requires some explanation. In effect, the conductance of a channel that is modelled by the GHK flux equation varies with voltage (see below figure) with a dependence on the ‘outer’ and ‘inner’ concentrations of the ion (in fact conductance is only constant with voltage when these concentrations are equal), as well as weakly on temperature.
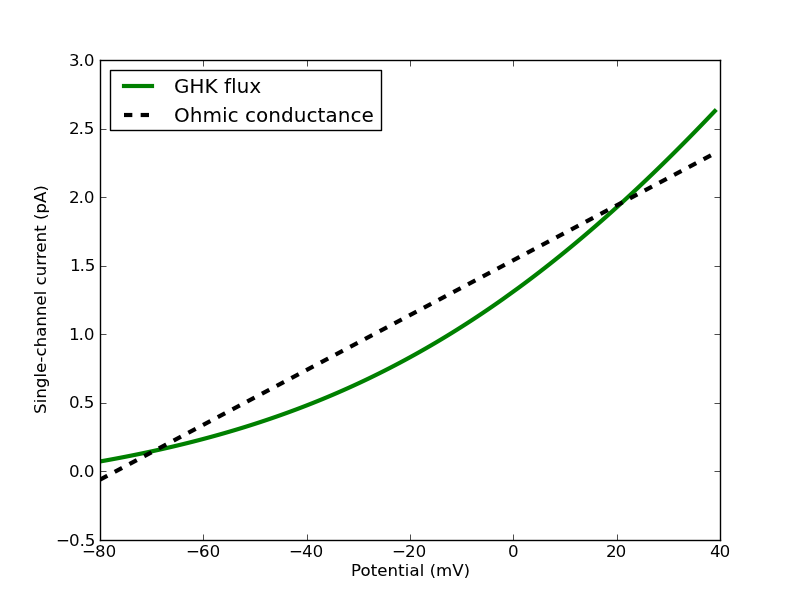
Figure 1: A single-channel GHK flux in the physiological range for a typical monovalent cation compared to an Ohmic approximation. The GHK flux is calculated with single-channel permeability of 9e-20 cubic metres / second, fixed extracellular concentration of 4mM, fixed intracellular concentration of 155mM and temperature of 20 Celsius. The single-channel Ohmic conductance is 20pS with reversal potential -77mV.
STEPS is able to estimate single-channel permeability from single-channel conductance, but for STEPS to do so the user must supply information about the conditions under which the conductance was measured, and in theory this should be enough to find the single-channel permeability since it is assumed constant (although there are occasions when permeability too can have some weak voltage dependence 3, which is, however, currently not possible to model with STEPS). Specifically,
the steps.API_2.model.GHKCurr.PInfo function requires arguments of: estimated single-channel conductance 5 (units: Siemens), one voltage within the range at which conductance was measured (Volts), temperature (Kelvin), ‘outer’ concentration of the ion (molar), and ‘inner’ concentration of the ion (molar). Since the valence of the ion is known it is not necessary to supply that information to the
steps.API_2.model.GHKCurr.PInfo function. So, for example, for some GHKcurrent object called K_GHK, if we measured single-channel conductance as 20pS in a small voltage range around -22mV at 20 degrees Celsius (293.15 Kelvin) with an estimated extracellular ion concentration of 4mM and intracellular concentration of 155mM, then we could create the current with
K_Pinfo = GHKCurr.PInfo(g = 20e-12, V = -22e-3, T = 293.15, oconc = 4e-3, iconc = 155e-3)
K_GHK = GHKCurr.Create(Kchan[Ko], K, K_Pinfo)
and the single-channel permeability would be set to approximately 9e-20 cubic metres / second. The behaviour of such a channel is shown in Figure 1.
We are now familiar, through aspects discussed so far in this chapter and other chapters, with most of the concepts applied for this model, so a very detailed description is not necessary for most remaining parts of the model. We move on to our other three ion channels in the model.
11.2.3. T-type Calcium channel
Like the P-type Calcium channel, transitions between channel states of the T-type Calcium channel are voltage-dependent and we model the calcium current as a GHK current
[9]:
with mdl:
with ssys:
# CaT channel
with CaTchan[...]:
CaTmc.s <r[1]> CaTmo.s
r[1].K = alpham_cat, betam_cat
CaThc.s <r[1]> CaTho.s
r[1].K = alphah_cat, betah_cat
OC_CaT = GHKCurr.Create(
CaTchan[CaTmo, CaTmo, CaTho], Ca, CaT_P,
computeflux=True,
virtual_oconc=Ca_oconc,
)
11.2.4. BK-type Calcium-activated Potassium channel
The BK channel in the model undergoes both voltage-dependent and non-voltage dependent processes. This is an example of Channel States interacting with Species through surface reactions. Here we will notice that Channel subunit states (e.g. BK) appear alongside Species (Ca) in reactions:
[10]:
with mdl:
with ssys:
# BK channel
with BKchan[..., BKclose]:
BK.s + Ca.i <r[1]> BKCa.s
r[1].K = BK_f, BKc_b
with BKchan[..., BKopen]:
BK.s + Ca.i <r[1]> BKCa.s
r[1].K = BK_f, BKo_b
with BKchan[...]:
BKclose.s <r[1]> BKopen.s
BK_f = CompDepRate.Create(lambda s: BK_oc_f[s.Count(BKCa)], [BKchan])
BK_b = CompDepRate.Create(lambda s: BK_oc_b[s.Count(BKCa)], [BKchan])
r[1].K = BK_f, BK_b
OC_BK = OhmicCurr.Create(BKchan[..., BKopen], BK_G, BK_rev)
The potassium current is modeled with an steps.API_2.model.OhmicCurr objects. All states in which the BKocSU subunit is in the BKopen state are conducting states, demonstrating the support for multiple conducting/permeable states for a channel.
Note also that the transition between BKclose and BKopen is declared with a complex-dependent rate (CompDepRate). As we saw in the multi-state complexes chapter, the rate of the reaction will be determined depending on the state of the channel. Here, the rate depends on the number of BKCaSU subunits that are bound to calcium. We declared tables of reaction rate constants
(BK_oc_f and BK_oc_b) and we access the correct value by counting the number of subunits that have bound calcium (BK_oc_f[s.Count(BKCa)]).
11.2.5. SK-type Calcium-activated Potassium channel
The SK channel does not have any voltage dependence, and contains two conducting states
[11]:
with mdl:
with ssys:
# SK channel
with SKchan[...]:
((SK_C1.s + Ca.i <r[1]> SK_C2.s)\
+ Ca.i <r[2]> SK_C3.s)\
+ Ca.i <r[3]> SK_C4.s
r[1].K = dirc2_t, invc1_t
r[2].K = dirc3_t, invc2_t
r[3].K = dirc4_t, invc3_t
SK_C3.s <r[1]> SK_O1.s
SK_C4.s <r[2]> SK_O2.s
r[1].K = diro1_t, invo1_t
r[2].K = diro2_t, invo2_t
OC_SK = OhmicCurr.Create(SKchan[SK_O1|SK_O2], SK_G, SK_rev)
11.2.6. Leak channel
Although another option for setting the leak would have been to (later) set sim.membrane.Res, the leak conductance is described as a leak channel:
[12]:
with mdl:
with ssys:
# Leak current channel
OC_L = OhmicCurr.Create(L[Leak], L_G, L_rev)
11.3. Geometry specification
This model is set up with a relatively simple geometry: a cylinder of diameter 2um and 80um length. The membrane across which we compute potential does not include the ends of the cylinder.
[13]:
###########################################################
# Mesh and compartmentalization
###########################################################
mesh = TetMesh.LoadGmsh(mesh_file, 1e-6)
with mesh:
cyto = Compartment.Create(mesh.tets, vsys)
ends = [mesh.bbox.min.z, mesh.bbox.max.z]
memb_tris = TriList(tri for tri in mesh.surface if tri.center.z not in ends)
memb = Patch.Create(memb_tris, cyto, None, ssys)
submemb_tets = TetList()
for tri in memb.tris:
submemb_tets |= tri.tetNeighbs
membrane = Membrane.Create([memb])
We also find the submembrane tetrahedrons, that is all tetrahedrons connected to a membrane triangle from the intracellular side.
11.4. Simulation with TetOpSplit
11.4.1. Initialization
We first create the random number generator, the partition scheme, and the simulation object:
[14]:
###########################################################
# Simulation
###########################################################
rng = RNG('mt19937', 512, SEED)
part = LinearMeshPartition(mesh, 1, 1, MPI.nhosts)
sim = Simulation('TetOpSplit', mdl, mesh, rng, part, calcMembPot=True)
Model checking:
No errors were found
We then declare the data to be saved, as seen in previous chapters:
[15]:
rs = ResultSelector(sim)
Currents = rs.SUM(rs.TRIS(memb.tris).OC_CaP.I) << \
rs.SUM(rs.TRIS(memb.tris).OC_CaT.I) << \
rs.SUM(rs.TRIS(memb.tris).OC_BK.I) << \
rs.SUM(rs.TRIS(memb.tris).OC_SK.I)
Pot = rs.TET(0, 0, 0).V
CaConcs = rs.cyto.Ca.Conc << \
(rs.SUM(rs.TETS(submemb_tets).Ca.Count) / (AVOGADRO * submemb_tets.Vol * 1e3))
BKstates = rs.memb.LIST(*BKchan[...]).Count
sim.toSave(Currents, Pot, CaConcs, BKstates, dt=DT)
We will save the total currents through the membrane for each GHK and ohmic currents, the membrane potential, the intracellular and submembrane calcium concentration, and the distribution of states of the BK channel.
The data will be saved to an HDF5 file, as we saw in a previous chapter. We setup the initial state for each run and run NBRUNS simulations:
[ ]:
with HDF5Handler('Caburst') as hdf:
sim.toDB(hdf, 'CaBurstSim')
for i in range(NBRUNS):
sim.newRun()
# Setting initial conditions
area = Parameter(memb.Area, 'm^2')
sim.memb.Pump.Count = round(P_ro * area)
for s in CaPchan[...]:
sim.memb.LIST(s).Count = round(CaP_ro*area*CaP_p[s.Count(CaPo)])
for s in CaTchan[...]:
hCnt, mCnt = s.Count(CaTho), s.Count(CaTmo)
sim.memb.LIST(s).Count = round(CaT_ro*area*CaT_p[hCnt][mCnt])
for s in BKchan[...]:
isOpen, nbCa = s.Count(BKopen), s.Count(BKCa)
sim.memb.LIST(s).Count = round(BK_ro*area*BK_p[isOpen][nbCa])
sim.memb.SKchan[SK_C1].Count = round(SK_ro*area*SK_C1_p)
sim.memb.SKchan[SK_C2].Count = round(SK_ro*area*SK_C2_p)
sim.memb.SKchan[SK_C3].Count = round(SK_ro*area*SK_C3_p)
sim.memb.SKchan[SK_C4].Count = round(SK_ro*area*SK_C4_p)
sim.memb.SKchan[SK_O1].Count = round(SK_ro*area*SK_O1_p)
sim.memb.SKchan[SK_O2].Count = round(SK_ro*area*SK_O2_p)
sim.memb.L[Leak].Count = round(L_ro * area)
sim.cyto.Ca.Conc = Ca_iconc
sim.cyto.Mg.Conc = Mg_conc
sim.cyto.CB[CBs, CBf, CBimmob].Conc = iCBsf_conc
sim.cyto.CB[CBsCa, CBf, CBimmob].Conc = iCBCaf_conc
sim.cyto.CB[CBs, CBfCa, CBimmob].Conc = iCBsCa_conc
sim.cyto.CB[CBsCa, CBfCa, CBimmob].Conc = iCBCaCa_conc
sim.cyto.CB[CBs, CBf, CBmob].Conc = CBsf_conc
sim.cyto.CB[CBsCa, CBf, CBmob].Conc = CBCaf_conc
sim.cyto.CB[CBs, CBfCa, CBmob].Conc = CBsCa_conc
sim.cyto.CB[CBsCa, CBfCa, CBmob].Conc = CBCaCa_conc
sim.cyto.PV.Conc = PV_conc
sim.cyto.PVCa.Conc = PVCa_conc
sim.cyto.PVMg.Conc = PVMg_conc
sim.EfieldDT = EF_DT
sim.ALL(Membrane).Potential = init_pot
sim.membrane.VolRes = Ra
sim.membrane.Capac = memb_capac
# Set temperature for ghk reactions
sim.Temp = TEMPERATURE
for j in range(1000):
t = ENDT * j / 999
if MPI.rank == 0:
print(f'run {i}: {t} / {ENDT}s')
sim.run(t)
We set a new simulation property, steps.API_2.sim.Simulation.Temp, which holds the simulation temperature. Currently, this will only influence any GHK flux rates, and will have no influence on any other kinetics. The value for TEMPERATURE is in Kelvin and corresponds to 34 degrees Celsius.
Note that, since the simulation takes much more computing time than other simulations we saw in previous chapters, we split the call to sim.run(ENDT) to multiple calls interspersed with printouts giving us feedback about the progression of the simulation.
11.4.2. Running the simulation
Although the simulation can be run with jupyter notebook, it would take too long to do so and it is thus more suited to parallel runs (see corresponding chapter). The corresponding python script can be downloaded here: STEPS_Tutorial_CaBurst.py The simulations can then be run with 12 cores by using e.g.:
mpirun -n 12 python3 STEPS_Tutorial_CaBurst.py
11.5. Parameters export
Finally, after the simulations, we call the ExportParameters function to generate parameter tables for our model and simulation:
[ ]:
if MPI.rank == 0:
ExportParameters(sim, 'CaBurst', method='pdf',
hideColumns=['Defined in', 'valence Units'],
unitsToSimplify=[
'uM', 'uM^-1 s^-1', 'mV', 'um^2 s^-1', 'pS', 'um', 'um^2'
], numPrecision=5,
)
First note that only MPI rank 0 calls this function, otherwise all ranks would try to write to the same files.
This function takes a STEPS container object as first argument (here we use the full simulation) and a file prefix as second argument. It will then generate files like CaBurst_Parameters.pdf, etc.
The method keyword argument specifies how the tables will be created, currently 'csv', 'tex' and 'pdf' are supported. The 'pdf' method depends on the installation of pdflatex and csv2latex programs. If these programs are not installed, you can change method='pdf' to method='csv' in the code above.
We will not go over all the available keyword arguments for the ExportParameters function, further explanations are available in the documentation. The following figure shows some of the tables generated by the function:
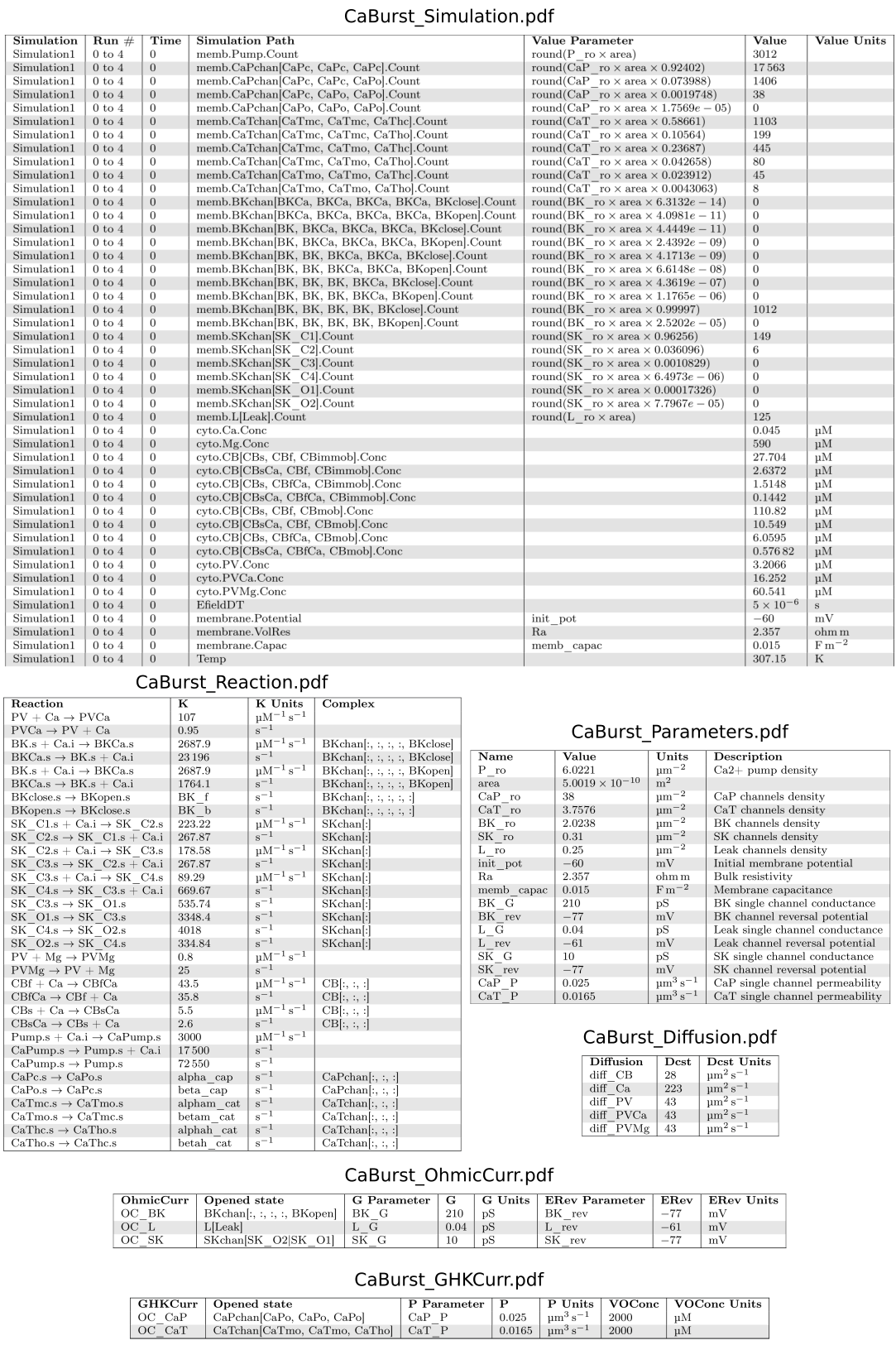
Figure 2: Examples of tables generated with ExportParameters. The simulation table shows the initial state (t = 0) values along with computations that were used to derive them. The reaction table shows all reactions in the model with their associated rate constants. The parameters table shows all explicitely created Parameters along with their description. The diffusion, GHKCurr and OhmicCurr tables respectively show diffusion rules, GHK currents and ohmic currents.
11.6. Plotting the results
The corresponding python script: STEPS_Tutorial_CaBurst_plot.py
[16]:
from matplotlib import pyplot as plt
import numpy as np
with HDF5Handler('Caburst') as hdf:
Currents, Pot, CaConcs, BKstates = hdf['CaBurstSim'].results
# Membrane potential
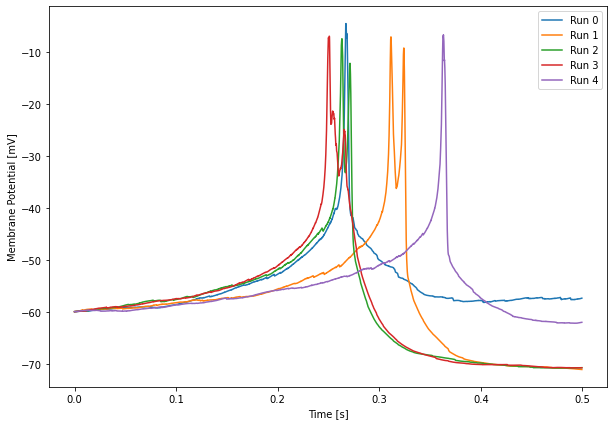
plt.figure(figsize=(10, 7))
for r in range(len(Pot.time)):
plt.plot(Pot.time[r], 1e3 * Pot.data[r,:,0], label=f'Run {r}')
plt.legend()
plt.xlabel('Time [s]')
plt.ylabel('Membrane Potential [mV]')
plt.show()
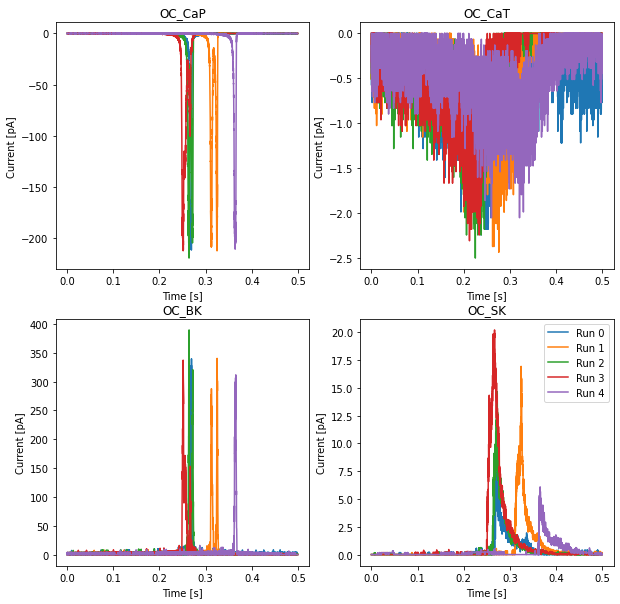
# Currents
fig, axs = plt.subplots(2, 2, figsize=(10, 10))
for i in range(len(Currents.data[0,0,:])):
ax = axs[i//2][i%2]
for r in range(len(Currents.time)):
ax.plot(Currents.time[r], 1e12 * Currents.data[r,:,i], label=f'Run {r}')
ax.set_title(Currents.labels[i].split('.')[-2])
ax.set_xlabel('Time [s]')
ax.set_ylabel('Current [pA]')
plt.legend()
# Calcium
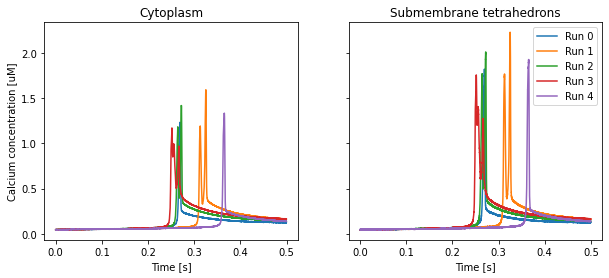
fig, axs = plt.subplots(1, 2, squeeze=True, sharey=True, figsize=(10, 4))
for i in range(len(CaConcs.data[0,0,:])):
for r in range(len(CaConcs.time)):
axs[i].plot(CaConcs.time[r], 1e6 * CaConcs.data[r,:,i], label=f'Run {r}')
axs[i].set_title(['Cytoplasm', 'Submembrane tetrahedrons'][i])
axs[i].set_xlabel('Time [s]')
if i == 0:
axs[i].set_ylabel('Calcium concentration [uM]')
plt.legend()
plt.show()
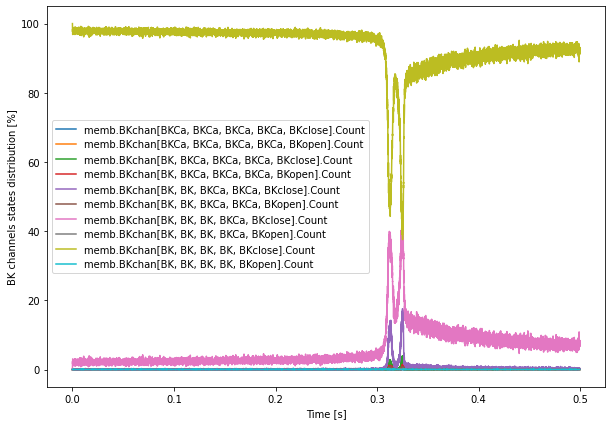
# BK channel states for run 1
rind = 1
plt.figure(figsize=(10, 7))
totCount = np.sum(BKstates.data[rind,:,:], axis=1)
for c in range(len(BKstates.data[rind, 0, :])):
plt.plot(BKstates.time[rind], 100 * BKstates.data[rind,:,c] / totCount)
plt.legend(BKstates.labels)
plt.xlabel('Time [s]')
plt.ylabel('BK channels states distribution [%]')
plt.show()
11.7. Footnotes
Anwar H, Hepburn I, Nedelescu H, Chen W, De Schutter E (2013) Stochastic Calcium Mechanisms Cause Dendritic Calcium Spike Variability. The Journal of Neuroscience, 33(40): 15848-15867, doi: 10.1523/JNEUROSCI.1722-13.2013.
Anwar H, Hong S, De Schutter E (2012) Controlling Ca2+-activated K+ channels with models of Ca2+ buffering in Purkinje cells. Cerebellum, 11(3):681-93, doi: 10.1007/s12311-010-0224-3.
Hepburn I, Cannon R and De Schutter E (2013) Efficient calculation of the quasi-static electrical potential on a tetrahedral mesh and its implementation in STEPS. Frontiers in Computational Neuroscience: 7:129, doi: 10.3389/fncom.2013.00129
The same considerations for converting membrane permeability to single-channel permeability apply as for conductance discussed in Simulating membrane potentia, requiring some estimate of the channel density.
Since it is assumed that conductance is measured by estimating the slope of an I-V curve over some small voltage range, the conductance will be treated as a slope conductance for the purposes of single-channel permeability estimation.
We may record voltage from anywhere on the membrane surface or within the ‘conduction volume’ (here and in most models the conduction volume is the cytosolic compartment).